- FDA-cleared
- Safe and effective
- Non-drug and non-invasive
- Covered by most insurances
Give your patients with MDD, OCD, Anxious Depression, and Adolescent Depression (15-21) proven relief that lasts.
Call us today 855-494-4867
Call us today 855-494-4867

Give your patients with MDD, OCD, Anxious Depression, and Adolescent Depression (15-21) proven relief that lasts.
Treat Depression at the Source
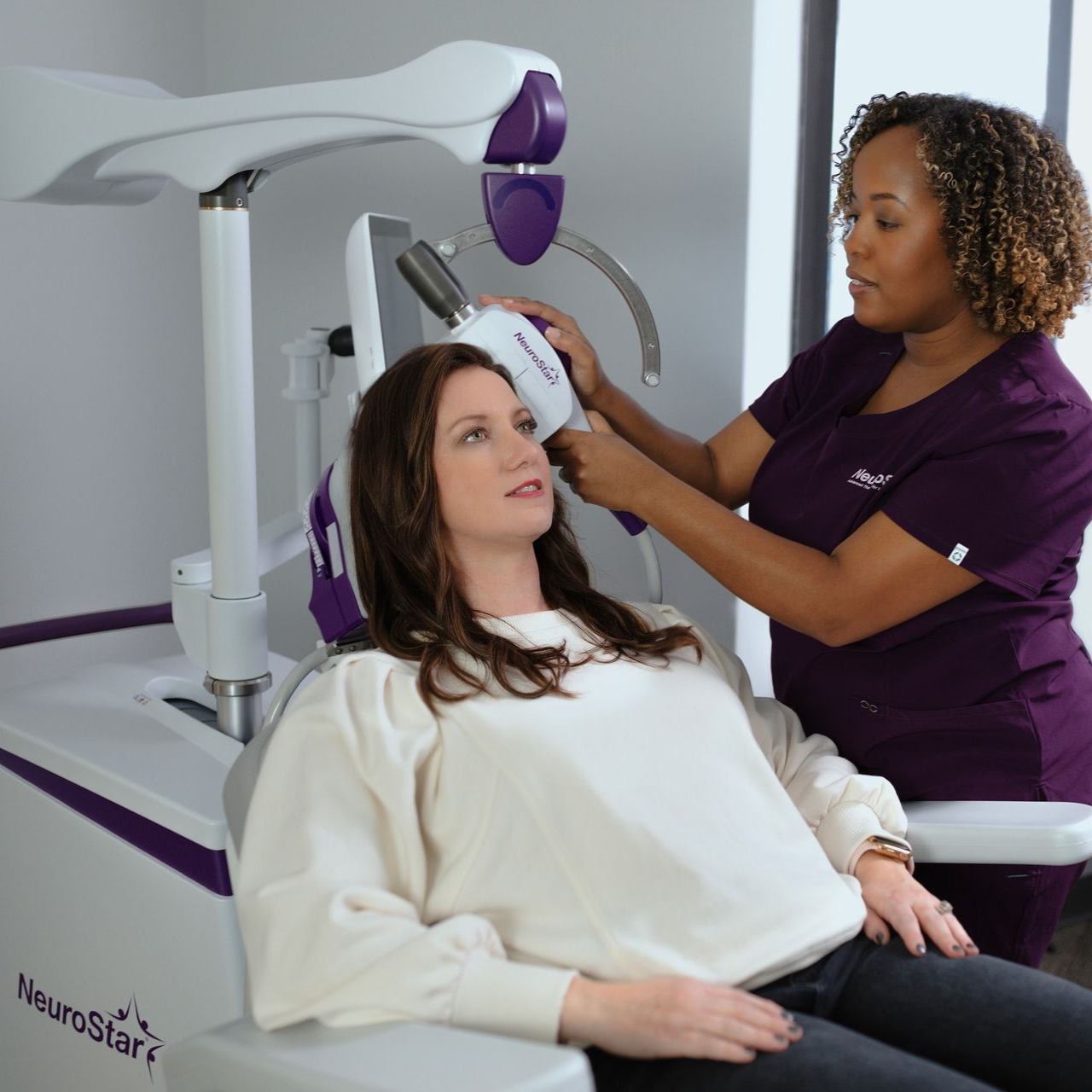
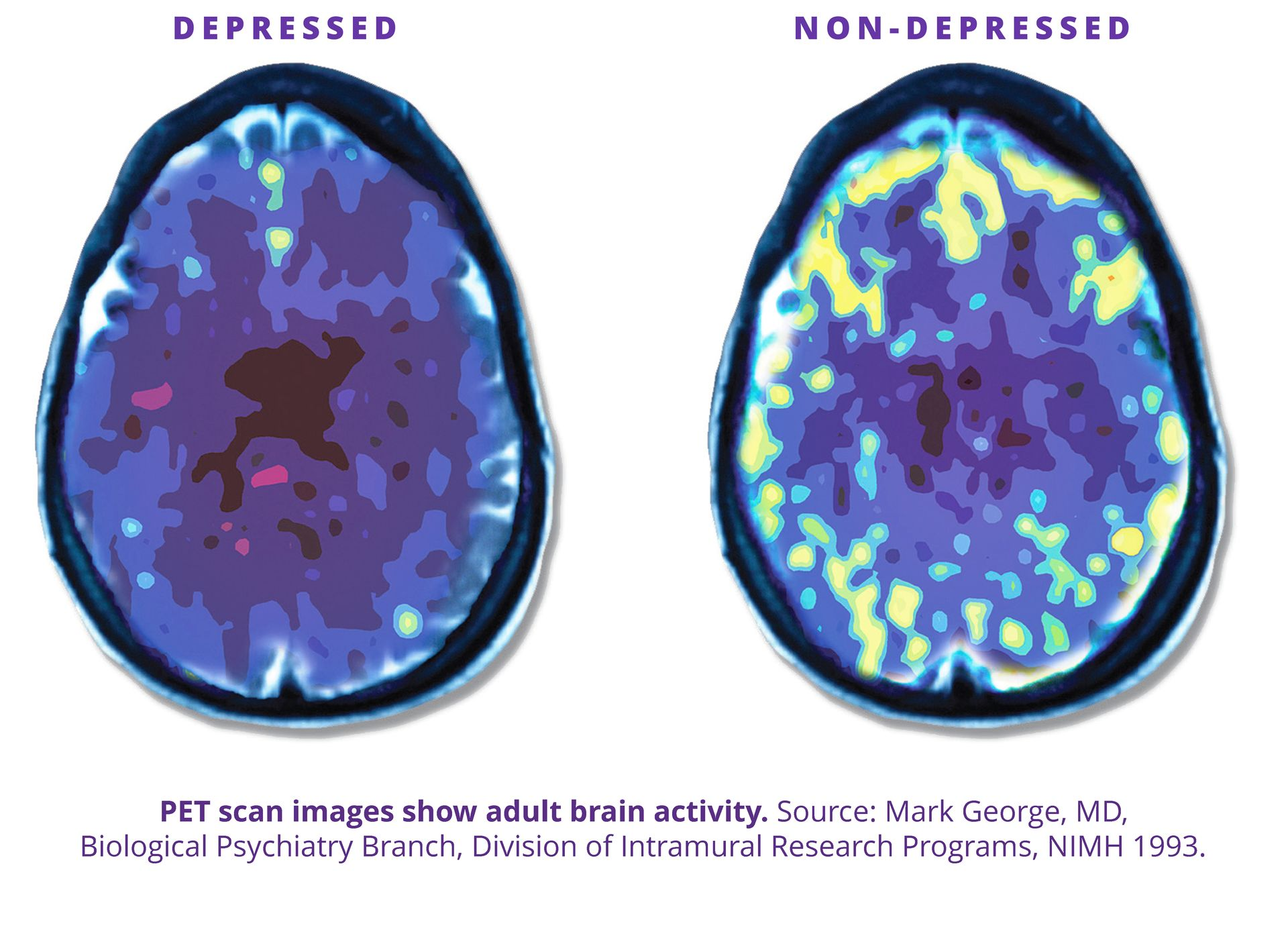
NeuroStar TMS (Transcranial Magnetic Stimulation) goes right to the source of depression – the brain. It is a non-invasive, non-drug treatment that uses focused magnetic pulses to “wake up” those areas, and help patients’ brain work the way it should. 5,6 It’s like physical therapy for the brain.
The #1 Physician Recommended TMS Treatment
Since 2008, NeuroStar has been used over 6.9 million times in over 188,000 patients, proving to be safe and effective for adults and adolescents (15-21) with depression.18
Long-Lasting Results
NeuroStar is the only treatment clinically proven to be effective through 12 months in adult treatment-resistant depression (TRD) patients.8
NeuroStar is an Easy, In-Office Experience
Patients are awake and fully alert during treatment.
* The NeuroStar Healthcare Provider will determine what is right for the patient.
Identifying a Potential NeuroStar TMS Candidate
Greenbrook supports an accessible internet. If you have any questions about our accessibility features, please contact us at
(855) 494-4867 and/or info@greenbrooktms.com.
NeuroStar Adult Indications for Use
The NeuroStar Advanced Therapy System is indicated for the treatment of depressive episodes and for decreasing anxiety symptoms for those who may exhibit comorbid anxiety symptoms in adult patients suffering from Major Depressive Disorder (MDD) and who failed to achieve satisfactory improvement from previous antidepressant medication treatment in the current episode.
The NeuroStar Advanced Therapy System is intended to be used as an adjunct for the treatment of adult patients suffering from Obsessive-Compulsive Disorder (OCD).
NeuroStar Adolescent Indications for Use
NeuroStar Advanced Therapy is indicated as an adjunct for the treatment of Major Depressive Disorder (MDD) in adolescent patients (15-21).
Important Safety Information
NeuroStar Advanced Therapy is only available by prescription. A doctor can help decide if NeuroStar Advanced Therapy is right for you. Patients’ results may vary.
The most common side effect is pain or discomfort at or near the treatment site. These events are transient; they occur during the TMS treatment course and do not occur for most patients after the first week of treatment. There is a rare risk of seizure associated with the use of TMS therapy (<0.1% per patient).
Visit neurostar.com for full safety and prescribing information.
Important Safety Information
WARNING: SEDATION; DISSOCIATION; RESPIRATORY DEPRESSION; ABUSE AND MISUSE; and SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning
CONTRAINDICATIONS
SPRAVATO® is contraindicated in patients with:
WARNINGS AND PRECAUTIONS
Sedation: SPRAVATO® may cause sedation or loss of consciousness. In some cases, patients may display diminished or less apparent breathing. In clinical trials, 48% to 61% of SPRAVATO®-treated patients developed sedation and 0.3% to 0.4% of SPRAVATO®-treated patients experienced loss of consciousness.
Because of the possibility of delayed or prolonged sedation, patients must be monitored by a healthcare provider for at least 2 hours at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting.
Closely monitor for sedation with concomitant use of SPRAVATO® with CNS depressants (e.g., benzodiazepines, opioids, alcohol).
Dissociation: The most common psychological effects of SPRAVATO® were dissociative or perceptual changes (including distortion of time, space and illusions), derealization and depersonalization (61% to 84% of SPRAVATO®-treated patients developed dissociative or perceptual changes). Given its potential to induce dissociative effects, carefully assess patients with psychosis before administering SPRAVATO®; treatment should be initiated only if the benefit outweighs the risk.
Because of the risks of dissociation, patients must be monitored by a healthcare provider for at least 2 hours at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting.
Respiratory Depression: In postmarketing experience, respiratory depression was observed with the use of SPRAVATO®. In addition, there were rare reports of respiratory arrest.
Because of the risks of respiratory depression, patients must be monitored for changes in respiratory status by a healthcare provider for at least 2 hours (including pulse oximetry) at each treatment session, followed by an assessment to determine when the patient is considered clinically stable and ready to leave the healthcare setting.
Abuse and Misuse: SPRAVATO® contains esketamine, a Schedule III controlled substance (CIII), and may be subject to abuse and diversion. Assess each patient’s risk for abuse or misuse prior to prescribing and monitor all patients for the development of these behaviors or conditions, including drug-seeking behavior, while on therapy. Individuals with a history of drug abuse or dependence are at greater risk; therefore, use careful consideration prior to treatment of individuals with a history of substance use disorder and monitor for signs of abuse or dependence.
SPRAVATO® Risk Evaluation and Mitigation Strategy (REMS): SPRAVATO® is available only through a restricted program called the SPRAVATO® REMS because of the risks of serious adverse outcomes from sedation, dissociation, respiratory depression, and abuse and misuse.
Important requirements of the SPRAVATO® REMS include the following:
Further information, including a list of certified pharmacies, is available at www.SPRAVATOrems.com/ or 1-855-382-6022.
Suicidal Thoughts and Behaviors in Adolescents and Young Adults: In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included adult and pediatric patients, the incidence of suicidal thoughts and behaviors in patients age 24 years and younger was greater than in placebo- treated patients. SPRAVATO® is not approved in pediatric (<18 years of age) patients.
There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing SPRAVATO® and/or the concomitant oral antidepressant, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
Increase in Blood Pressure: SPRAVATO® causes increases in systolic and/or diastolic blood pressure (BP) at all recommended doses. Increases in BP peak approximately 40 minutes after SPRAVATO® administration and last approximately 4 hours. Approximately 3% to 19% of SPRAVATO®-treated patients experienced an increase of more than 40 mmHg in systolic BP and/or 25 mmHg in diastolic BP
in the first 1.5 hours after administration at least once during the first 4 weeks of treatment. A substantial increase in blood pressure could occur after any dose administered even if smaller blood pressure effects were observed with previous administrations. SPRAVATO® is contraindicated in patients for whom an increase in BP or intracranial pressure poses a serious risk (e.g., aneurysmal vascular disease, arteriovenous malformation, history of intracerebral hemorrhage). Before prescribing SPRAVATO®, patients with other cardiovascular and cerebrovascular conditions should be carefully assessed to determine whether the potential benefits of SPRAVATO® outweigh its risk.
Assess BP prior to administration of SPRAVATO®. In patients whose BP is elevated prior to SPRAVATO® administration (as a general guide: >140/90 mmHg), a decision to delay SPRAVATO® therapy should take into account the balance of benefit and risk in individual patients.
BP should be monitored for at least 2 hours after SPRAVATO® administration. Measure blood pressure around 40 minutes post-dose and subsequently as clinically warranted until values decline. If BP remains high, promptly seek assistance from practitioners experienced in BP management. Refer patients experiencing symptoms of a hypertensive crisis (e.g., chest pain, shortness of breath) or hypertensive encephalopathy (e.g., sudden severe headache, visual disturbances, seizures, diminished consciousness, or focal neurological deficits) immediately for emergency care.
Closely monitor blood pressure with concomitant use of SPRAVATO® with psychostimulants (e.g., amphetamines, methylphenidate, modafinil, armodafinil) or monoamine oxidase inhibitors (MAOIs).
In patients with a history of hypertensive encephalopathy, more intensive monitoring, including more frequent blood pressure and symptom assessment, is warranted because these patients are at increased risk for developing encephalopathy with even small increases in blood pressure.
Cognitive Impairment
Short-Term Cognitive Impairment: In a study in healthy volunteers, a single dose of SPRAVATO® caused cognitive performance decline 40 minutes post-dose. Compared to placebo-treated subjects, SPRAVATO®-treated subjects required a greater effort to complete the cognitive tests at 40 minutes post-dose. Cognitive performance and mental effort were comparable between SPRAVATO® and placebo at 2 hours post-dose. Sleepiness was comparable after 4 hours post-dose.
Long-Term Cognitive Impairment: Long-term cognitive and memory impairment have been reported with repeated ketamine misuse or abuse. In 1-year and 3-year, long-term, open-label clinical trials in adults, the effect of SPRAVATO® on cognitive functioning remained stable over time as evaluated by the Cogstate computerized battery and Hopkins Verbal Learning Test-Revised.
Impaired Ability to Drive and Operate Machinery: Before SPRAVATO® administration, instruct patients not to engage in potentially hazardous activities requiring complete mental alertness and motor coordination, such as driving a motor vehicle or operating machinery, until the next day following a restful sleep. Patients will need to arrange transportation home following treatment with SPRAVATO®.
Ulcerative or Interstitial Cystitis: Cases of ulcerative or interstitial cystitis have been reported in individuals with long-term off-label use or misuse/abuse of ketamine. In clinical studies with SPRAVATO® nasal spray, there was a higher rate of lower urinary tract symptoms (pollakiuria, dysuria, micturition urgency, nocturia, and cystitis) in SPRAVATO®-treated patients than in placebo-treated patients. No cases of esketamine-related interstitial cystitis were observed in any of the studies, which involved treatment for up to a year.
Monitor for urinary tract and bladder symptoms during the course of treatment with SPRAVATO® and refer to an appropriate healthcare provider as clinically warranted.
PREGNANCY, EMBRYO-FETAL TOXICITY, AND LACTATION
SPRAVATO® is not recommended during pregnancy. SPRAVATO® may cause fetal harm when administered to pregnant women. Advise pregnant women of the potential risk to an infant exposed to SPRAVATO® in utero. Advise women of reproductive potential to consider pregnancy planning and prevention.
There are risks to the mother associated with untreated depression in pregnancy. If a woman becomes pregnant while being treated with SPRAVATO®, treatment with SPRAVATO® should be discontinued and the patient should be counseled about the potential risk to the fetus.
Pregnancy Exposure Registry: There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including SPRAVATO®, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or online at https://womensmentalhealth.org/research/pregnancyregistry/antidepressants/.
SPRAVATO® is present in human milk. Because of the potential for neurotoxicity, advise patients that breastfeeding is not recommended during treatment with SPRAVATO®.
SELECT USE IN SPECIFIC POPULATIONS
Geriatric Use: No overall differences in the safety profile were observed between patients 65 years of age and older and patients younger than 65 years of age. At the end of a 4-week, randomized, double-blind study, there was no statistically significant difference between groups on the primary efficacy endpoint.
Hepatic Impairment: SPRAVATO®-treated patients with moderate hepatic impairment may need to be monitored for adverse reactions for a longer period of time.
SPRAVATO® has not been studied in patients with severe hepatic impairment (Child-Pugh class C). Use in this population is not recommended.
ADVERSE REACTIONS
TRD: The most commonly observed adverse reactions in patients treated with SPRAVATO® plus oral antidepressant (incidence ≥5% and at least twice that of placebo nasal spray plus oral antidepressant) were dissociation, dizziness, nausea, sedation, vertigo, hypoesthesia, anxiety, lethargy, blood pressure increased, vomiting, and feeling drunk.
Treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior: The most commonly observed adverse reactions in patients treated with SPRAVATO® plus oral antidepressant (incidence ≥5% and at least twice that of placebo nasal spray plus oral antidepressant) were dissociation, dizziness, sedation, blood pressure increased, hypoesthesia, vomiting, euphoric mood, and vertigo.
The most common adverse reactions with SPRAVATO® TRD monotherapy (≥5% and at least twice that of placebo nasal spray) were dissociation, nausea, dizziness, headache, anxiety, vomiting, feeling drunk, blood pressure increased, and sedation.
Indications:
SPRAVATO® (esketamine) CIII Nasal Spray is indicated for the treatment of:
Limitations of Use:
Please see full Prescribing Information, including Boxed WARNINGS, and Medication Guide for SPRAVATO®.
cp-499107v1
All Rights Reserved | Greenbrook TMS NeuroHealth Centers.
Indications:
SPRAVATO® (esketamine) CIII Nasal Spray is indicated for the treatment of:
Limitations of Use:
Please see full Prescribing Information, including Boxed WARNINGS, and Medication Guide for SPRAVATO®.
cp-499107v1